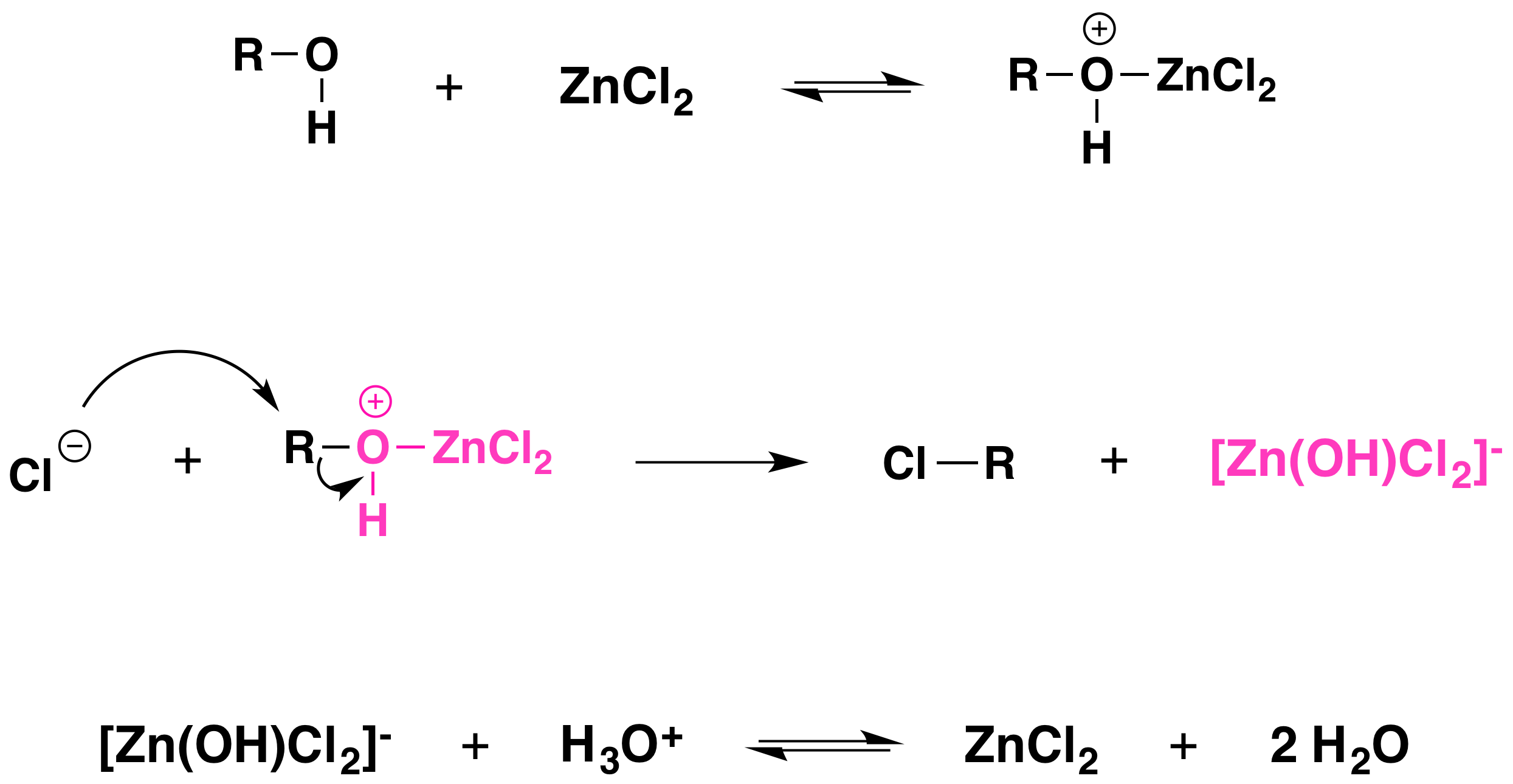Is Alcohol Very Acidic . Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. The conjugate acid of an alcohol is called an oxonium ion. Thus, sodium hydroxide (naoh) is not a strong enough base to. However, phenol is sufficiently acidic for it to.
from chem.libretexts.org
Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for it to. The conjugate acid of an alcohol is called an oxonium ion. Thus, sodium hydroxide (naoh) is not a strong enough base to. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides.
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts
Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. The conjugate acid of an alcohol is called an oxonium ion. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for it to. Thus, sodium hydroxide (naoh) is not a strong enough base to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions.
From chem.libretexts.org
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. Therefore, alcohols are more acidic than terminal alkynes,. Is Alcohol Very Acidic.
From dvn.com.vn
Meet the (Most Important) Functional Groups Chia Sẻ Kiến Thức Điện Is Alcohol Very Acidic Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is called an oxonium ion. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. However, phenol is sufficiently acidic for it to. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can. Is Alcohol Very Acidic.
From www.oculyze.net
The Beer Acidity Chart Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is. Is Alcohol Very Acidic.
From pediaa.com
Difference Between Lactic Acid and Alcoholic Fermentation Definition Is Alcohol Very Acidic Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually. Is Alcohol Very Acidic.
From advancedmixology.com
[Infographic] Best Least Acidic Wines For Those With Acid Reflux Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Thus, sodium hydroxide (naoh) is not a strong enough base to. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and. Is Alcohol Very Acidic.
From chem.libretexts.org
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. The conjugate acid of an alcohol is called an oxonium. Is Alcohol Very Acidic.
From chem.libretexts.org
Reactions of alcohols with hydrohalic acids (HX) Chemistry LibreTexts Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. However, phenol is sufficiently acidic for it to. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is called an oxonium ion. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can. Is Alcohol Very Acidic.
From www.youtube.com
Organic Chemistry Understanding Acidity of Alcohols YouTube Is Alcohol Very Acidic Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Thus, sodium hydroxide (naoh) is not a strong enough base to. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually. Is Alcohol Very Acidic.
From tastylicious.com
What's The Least Acidic Alcohol? Tastylicious Is Alcohol Very Acidic Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen. Is Alcohol Very Acidic.
From www.youtube.com
02.03 Acidity and Basicity of Alcohols YouTube Is Alcohol Very Acidic Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. The conjugate acid of an alcohol is called an oxonium ion. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen. Is Alcohol Very Acidic.
From chem.libretexts.org
Oxidation by Chromic Acid Chemistry LibreTexts Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. Therefore, alcohols are more acidic than terminal alkynes,. Is Alcohol Very Acidic.
From ar.inspiredpencil.com
Lactic Acid Fermentation And Alcoholic Fermentation Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. However, phenol is sufficiently acidic for it to. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is called an oxonium ion. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic. Is Alcohol Very Acidic.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID6508687 Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. The conjugate acid of an alcohol is called an oxonium ion. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Thus, sodium hydroxide (naoh) is not a strong enough base to. Alcohols are slightly weaker acids. Is Alcohol Very Acidic.
From shop.leicabiosystems.com
Acid Alcohol for H&E Staining, 1 & 0.5 Histology Consumables Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. The conjugate acid of an alcohol is called an oxonium ion. Thus, sodium hydroxide (naoh) is not a strong enough base to. However, phenol is sufficiently acidic. Is Alcohol Very Acidic.
From techiescientist.com
pH of Alcohol — Acidic or Alkaline? Techiescientist Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is called an oxonium ion. Alcohols are slightly weaker acids. Is Alcohol Very Acidic.
From www.slideserve.com
PPT Fermentation PowerPoint Presentation, free download ID2932291 Is Alcohol Very Acidic Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is. Is Alcohol Very Acidic.
From telegra.ph
What Exactly Are Some QuickActing Foods That Neutralize Gastric Acid Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases than hydroxide ions. However, phenol is sufficiently acidic for it to. The conjugate. Is Alcohol Very Acidic.
From www.nagwa.com
Question Video Understanding Acidity Differences between Alcohols and Is Alcohol Very Acidic Alcohols are so weakly acidic that, for normal lab purposes, their acidity can be virtually ignored. Thus, sodium hydroxide (naoh) is not a strong enough base to. The conjugate acid of an alcohol is called an oxonium ion. However, phenol is sufficiently acidic for it to. Alcohols are slightly weaker acids than water, and alkoxide ions are slightly stronger bases. Is Alcohol Very Acidic.